formula of magnesium oxide lab Michael Jansen reflects on a very common empirical formula lab that asks students to determine the empirical formula of MgxOy He then explains how he continues to use it as a successful failure how he
In the reaction of magnesium with atmospheric oxygen to produce magnesium oxide what are the coefficients of the following equation after it has been balanced Mg s O2 g In this experiment the percent composition and empirical formula of magnesium oxide the main compound that is formed when magnesium metal combines with oxygen in air will be determined Heating magnesium in the presence of air
formula of magnesium oxide lab
formula of magnesium oxide lab
https://studymoose.com/wp-content/uploads/essay-thumbnails/determining-the-empirical-formula-of-magnesium-oxide-lab-post-preview.webp

DOC IB Chemistry IA Determining The Empirical Formula Of Magnesium
https://0.academia-photos.com/attachment_thumbnails/34358970/mini_magick20180815-12926-w0836a.png?1534400147
Chem Matters Ch6 ionic bond
https://image.slidesharecdn.com/chemmattersch6ionicbond-120712101243-phpapp02/95/chem-matters-ch6ionicbond-31-728.jpg?cb=1342089001
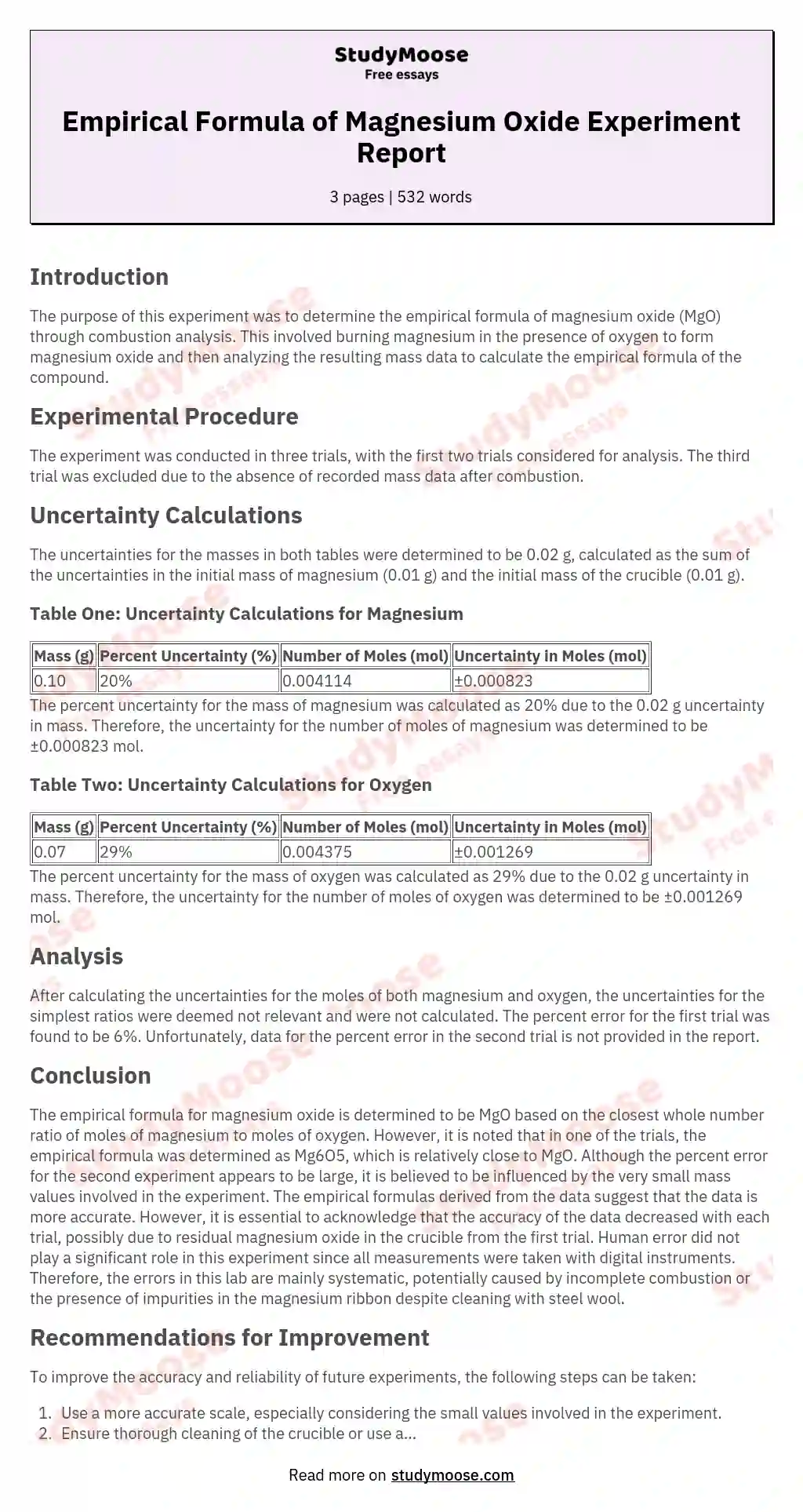
The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide From the masses of magnesium and oxygen that combine we can calculate the empirical formula of magnesium oxide We will weigh the magnesium before it combines with
The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence such as H2O for water or HO for hydrogen peroxide When The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO For today s
More picture related to formula of magnesium oxide lab

The Empirical Formula Of Magnesium Oxide Lab A Successful Failure
https://www.chemedx.org/sites/www.chemedx.org/files/images/blog/deanna-cullen/empirical-formula-magnesium-oxide-lab-successful-failure-next-steps—and-very-important-lesson.png
IS8025 Determination Of The Empirical Formula Of Magnesium Oxide
http://www.sciencelabsupplies.com/images/magictoolbox_cache_from_database/1b7f1df8ae54c4c23d8a23545c0993ae.jpg
DETERMINING THE EMPIRICAL FORMULA OF A COMPOUND Chegg
https://media.cheggcdn.com/media/0b9/0b949f79-f2d5-4aec-805e-90683704040e/image
The empirical formula of magnesium oxide is MgO Here is a video that illustrates how to determine an empirical formula The goal of this experiment is to determine the empirical formula of magnesium oxide experimentally Objectives 1 To burn a sample of magnesium in air and measure the gain in
In this lab we will determine the empirical formula of a compound by synthesizing a sample of that compound In a synthesisreaction a substance is created by mixing and reacting The experiment highlighted the formation of an ionic bond between a metal magnesium and a non metal oxygen to produce magnesium oxide The empirical formula

Empirical formula Of Magnesium Oxide Lab Report Answers The
https://media.cheggcdn.com/study/a7c/a7c4c45c-73e9-4f0b-b87b-a6e5c37402c9/image.png

Magnesium Oxide Percent Yield Lab Report SchoolWorkHelper
https://schoolworkhelper.net/wp-content/uploads/2020/03/mg-o-calculation.jpg
formula of magnesium oxide lab - From the masses of magnesium and oxygen that combine we can calculate the empirical formula of magnesium oxide We will weigh the magnesium before it combines with
