factors affecting solid pressure These stress explanations become much more difficult where one of the surfaces is not a solid So using large area tyres to move over mud or snowshoes to walk on snow are a little more complex than they seem to explain A first explanation will be along the same lines as for solid solid surfaces
This video is about pressure in solids and is for Key Stage Three pupils pupils in Years 7 8 This covers the pressure equation and gives examples of applying the pressure equation more Thrust And Pressure Atmospheric Pressure And Gauge Pressure Fluid Pressure Hydrostatic Pressure Factors Affecting Pressure Since the pressure is dependent on the area over which the force is acting the pressure can be increased and decreased without any change in the force
factors affecting solid pressure

factors affecting solid pressure
https://hi-static.z-dn.net/files/d3e/353378e3b28e4e196f3555225497e9c4.jpg

Pressure In Solids Explanation Examples YouTube
https://i.ytimg.com/vi/jWMsItc25b8/maxresdefault.jpg

Pressure In Solids KS3 Activate Science Teaching Resources
https://d1e4pidl3fu268.cloudfront.net/efc08d44-e5a2-4f40-8f67-28a16cbea786/TES.png
Learn about kinetic theory which includes using the Celsius and Kelvin scales the relationship between pressure temperature and volume in gas and energy changes when changes in state occur A solid surface can exert pressure but fluids i e liquids or gases can also exert pressure This might seem strange if you think about it because it s hard to imagine hammering in a nail with liquid To make sense of this imagine being submerged to
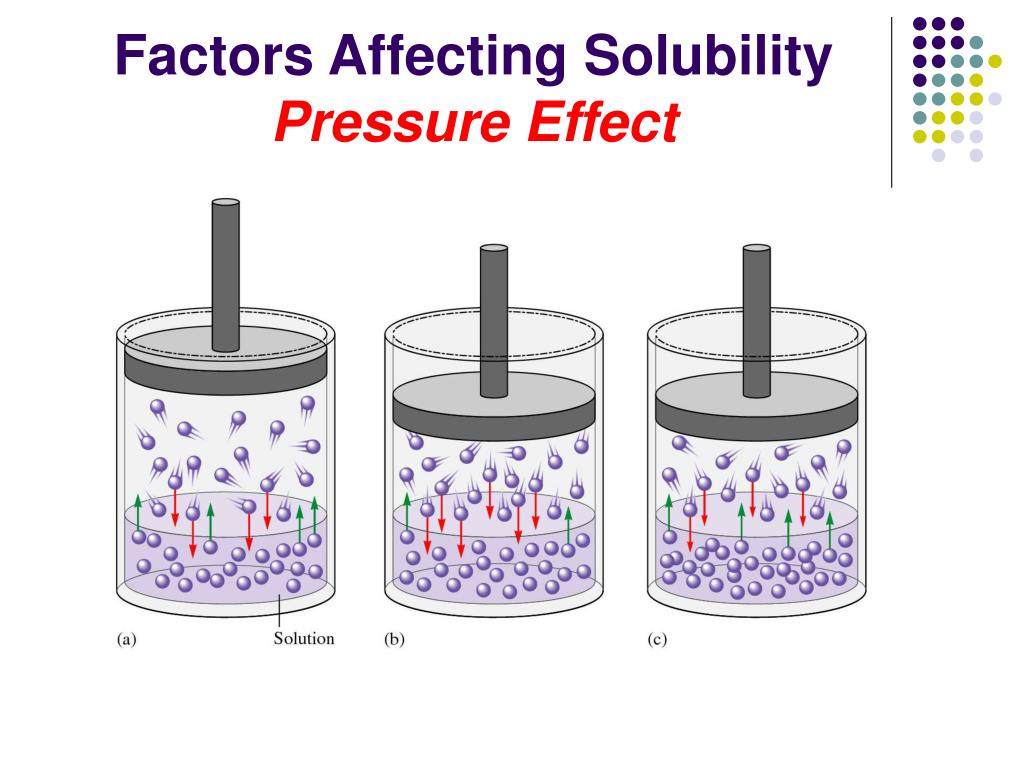
It may also be the result of static forces from the neighbouring atoms or molecules in the solid Since these forces can be attractive or repulsive in the current deformation state the pressure in a solid may also become negative tensile without necessarily tearing a vacuum bubble into the solid as opposed to a liquid or gas Factors Affecting Solubility The solubility of a solid or a liquid solute in a solvent is affected by the temperature while the solubility of a gaseous solute is affected by both the temperature and the pressure of the gas We will examine the effects of temperature and pressure separately
More picture related to factors affecting solid pressure
Schoolphysics Welcome
https://www.schoolphysics.co.uk/age11-14/glance/Matter/Pressure/images/1.gif

Welcome To CK 12 Foundation CK 12 Foundation
https://www.ck12.org/flx/show/image/201212141355507696282261_db6e64cb71e82ec7b65565de7435e6b3-201212141355507898498681.png
Reaction Rates When Surface Area Matters Lesson Plan
https://www.sciencebuddies.org/g5Eus4vmr6VLhyjh-4TTnN4piUQ=/590x306/-/https/www.sciencebuddies.org/Files/8864/10/lesson-plan-reaction-rates-surface-area.png
The propensity of a solid is to become more soluble as temperature goes up and for a gas to become less soluble We will revisit this in chapter 18 when we cover entropy and the second law of thermodynamics which essentially says that a process goes in the direction that increases the entropy of the universe that is how dispersed the The reason increasing pressure will increase the required temperature of changing the state is because the pressure is a result of molecules from the outside material like air molecules for air pressure smashing into the object s molecules and preventing them to become free from their own molecular attraction
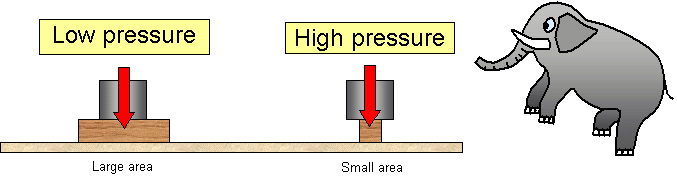
Example 1 Tractors Tractors have large tyres This spreads the weight force of the tractor over a large area This reduces the pressure which prevents the heavy tractor from sinking into the mud Example 2 Nails Nails have sharp pointed ends with a very small area This concentrates the force creating a large pressure over a small area The pressure in solids depends on different factors One of the most important parameters is the force applied on the solid As it increases the pressure also increases Moreover if area is increased it can lead to a reduction in the pressure
PPT Chapter 17 PowerPoint Presentation Free Download ID 956525
https://image.slideserve.com/956525/factors-affecting-solubility-pressure-effect-l.jpg
Factors Affecting Rate Of Reaction
https://www.cdli.ca/sampleResources/chem3202/unit01_org01_ilo03/u1s1l3_fig01.jpg
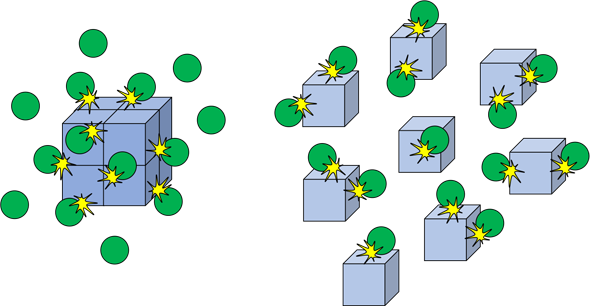
factors affecting solid pressure - The facts What happens Increasing the pressure on a reaction involving reacting gases increases the rate of reaction Changing the pressure on a reaction which involves only solids or liquids has no effect on the rate An example