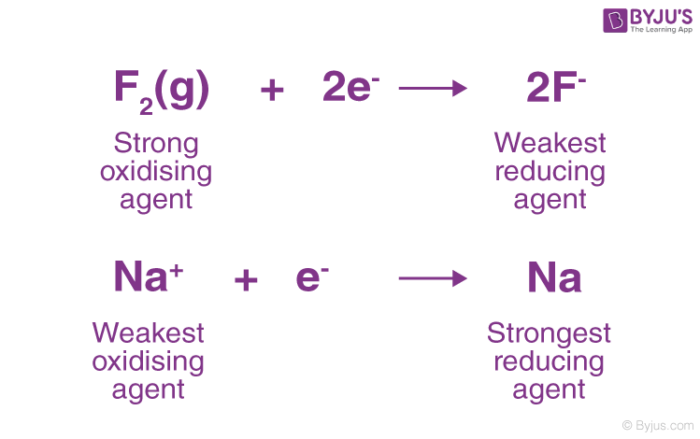
examples of reducing agents Sodium hydrogen and lithium are examples of strong oxidizing agents While weak reducing agents cannot lose electrons easily Fluorine chlorine iron etc are weak reducing agents We can know the strength of reducing agents by
A reducing agent is typically in one of its lower possible oxidation states and is known as the electron donor A reducing agent is oxidized because it loses electrons in the redox reaction Examples of reducing agents include the earth metals formic acid and sulfite compounds Examples of reducing agents include hydrogen gas alkali metals rare earth metals and compounds containing the hydride H anion A reducing agent loses electrons and is oxidized in a chemical reaction
examples of reducing agents
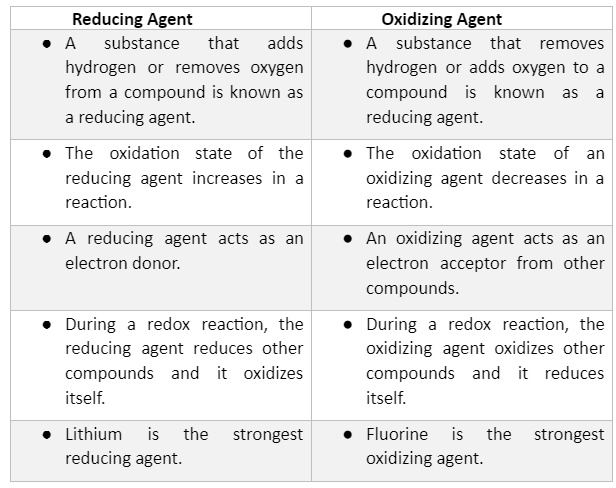
examples of reducing agents
https://unacademy.com/content/wp-content/uploads/sites/2/2022/04/7-29.png
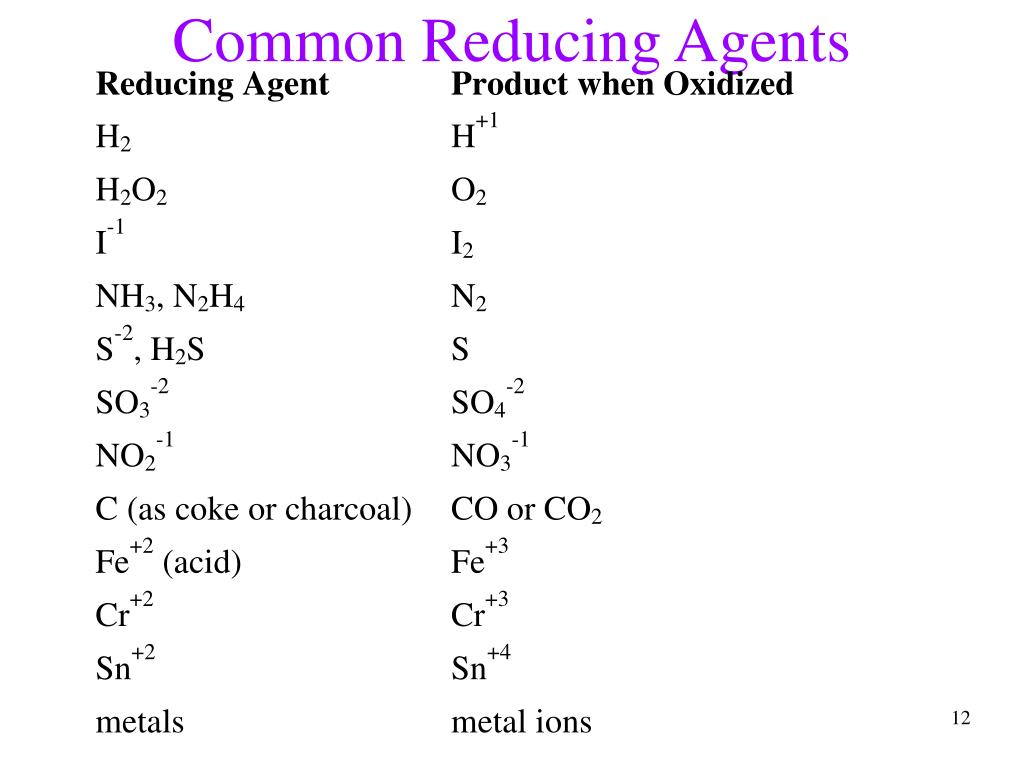
PPT Chapter 18 Electrochemistry PowerPoint Presentation Free
https://image.slideserve.com/932862/common-reducing-agents-l.jpg

How To Find The Oxidizing And Reducing Agent YouTube
https://i.ytimg.com/vi/HFgcCTCj1J4/maxresdefault.jpg
Examples of substances that are common reducing agents include hydrogen the alkali metals formic acid oxalic acid and sulfite compounds In their pre reaction states reducers have extra electrons that is they are by themselves reduced and oxidizers lack electrons that is they are by themselves oxidized Among the elements low electronegativity is characteristic of good reducing agents Molecules and ions which contain relatively electropositive elements which have low oxidation numbers are also good reducing agents Bear these general rules in mind as we examine examples of common reducing agents in the following paragraphs
Some common examples of reducing agents include metals such as zinc and iron as well as certain chemicals like sodium borohydride and hydrogen gas These substances are often used in industrial processes such as the production of metals and the reduction of organic compounds Common Reducing Agents When performing experiments it is undoubtedly useful to know what reducing agents you can use for your reaction The staff at ChemTalk features the commonly used reducing agents to inform your own hands on work at the bench List of Reducing Agents Ascorbic Acid Glucose
More picture related to examples of reducing agents
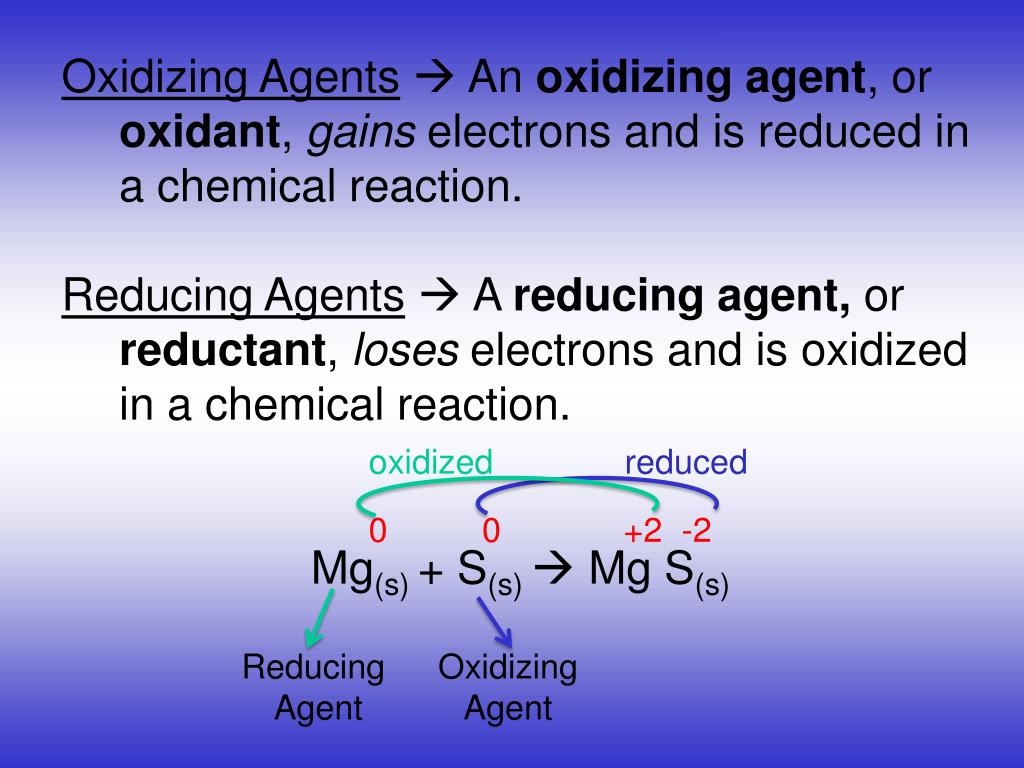
PPT Oxidizing Reducing Agents PowerPoint Presentation Free
https://image2.slideserve.com/4271025/slide2-l.jpg

Reducing Agent Reductant Definition Examples With Videos
https://cdn1.byjus.com/wp-content/uploads/2018/11/chemistry/wp-content/uploads/2016/10/word-image-18.png

Oxidizing And Reducing Agents Chemistry Libretexts Vrogue co
https://chemistrytalk.org/wp-content/uploads/2021/03/New-Common-Oxidizing-Agents-3-2.jpg
Three commonly used reducing agents are carbon in coke or coal carbon monoxide and methane These react with water vapor form H 2 g C s 2H 2O g rightarrow CO g H 2 g nonumber How do I look at a compound and tell if it is a reducing agent or an oxidizing agent For example sulfites and phosphites are reducing agents while permanganates and perchlorates are oxidizing agents but I DO NOT KNOW WHY
[desc-10] [desc-11]
Reducing Agent Reductant Definition Examples With Videos
https://cdn1.byjus.com/wp-content/uploads/2016/10/Reducing-Agent-2-700x439.png

What Is A Reducing Agent EliyenReese
https://i.ytimg.com/vi/wPvGa76xIEc/maxresdefault.jpg
examples of reducing agents - Examples of substances that are common reducing agents include hydrogen the alkali metals formic acid oxalic acid and sulfite compounds In their pre reaction states reducers have extra electrons that is they are by themselves reduced and oxidizers lack electrons that is they are by themselves oxidized