Entropy And Gibbs Free Energy Worksheet S7 E 2a The freezing of water is a decrease in entropy so it has the smallest Delta S because solids have less entropy than liquids The other two options represent an increase in Entropy The sublimation of ice is a large increase in Entropy because gas has more Entropy than solids The sublimation of ice to gas is more of an increase than
GIBB S FREE ENERGY PROBLEMS WORKSHEET Answer feel free to question my math Also sig figs The hydrogenation of ethene gas under standard conditions T 298 15 K shows a decrease in disorder S 0 1207 kJ mol K during an exothermic reaction H 136 9 kJ mol The change in the Gibbs free energy at 794 C assuming that the enthalpy and the entropy do not change at this temperature PCl5 g PCl3 g Cl2 g Calculate The change of enthalpy at 25 C The change of entropy at 25 C The change in the Gibbs free energy at 25 C
Entropy And Gibbs Free Energy Worksheet

Entropy And Gibbs Free Energy Worksheet
https://content.lessonplanet.com/resources/thumbnails/276133/original/odu4ndaxlnbuzw.png?1414473679

Chemistry Unit 13 Gibbs Free Energy Homework Pages Store Science
https://d2j6dbq0eux0bg.cloudfront.net/images/15443271/959738933.jpg

Gibbs Free Energy Worksheet
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/86c4f35503fa85cedcc7c38630538286/thumb_1200_1553.png
Gibb s free energy can be calculated from enthalpy change H and entropy change S randomness or degree of disorder G H T S Note denotes standard conditions 298 15 K 25 C and 1 atm 2 Negative ENTROPY and GIBBS FREE ENERGY Entropy is the degree of randomness in a substance The symbol for change in entropy is S Solids are very ordered and have low entropy
In general a significant increase in the entropy will occur if there is a change of state from solid or liquid to gas there is a significant increase in number of molecules between products and reactants NH4Cl s HCl g NH3 g S ve change from solid reactant to gaseous products WE 14 2 Entropy change of vaporization 3 on p 662 in Chemistry Calculate the entropy change when 1 00 mol of water at 0 C freezes to form ice For water fus Ho 6 02 kJ mol 1 Strategy Use Equation 14 6 which relates the entropy change for a system to the enthalpy change and the temperature Solution
More picture related to Entropy And Gibbs Free Energy Worksheet

Solved Chemistry 1225 Chapter 17 Worksheet Entropy And Chegg
https://media.cheggcdn.com/study/39d/39d39616-dbe9-443c-84bd-47b828788034/image.png

Solved Reaction Quotient and Gibbs free energy At The 9to5Science
https://i.stack.imgur.com/IqJ4W.jpg

Chemistry Blog Entropy Gibbs Free Energy Worksheet 4 10
https://lh5.googleusercontent.com/-XqrNZkGfm8E/U0a4SDWKDdI/AAAAAAAAAQ0/61rlps0Yusw/s640/blogger-image-1271629082.jpg
Exercise 18 6 1a Balance the following equations Calculate the change of the Gibbs free energy for the reaction at 25 C where standard free energy of formation of C 2 H 4 g H 2 O g C 2 H 5 OH l are 68 229 175 kJ mol respectively C2H4 g H2O g C2H5OH l What is the change of entropy if S 0 of C 2 H 4 g H 2 O g C 2 H Worksheet on Entropy and Free Energy 1 Define entropy in your own words and list the variables or conditions that you must consider when comparing the entropy of two substances or when trying to determine the relative change in entropy 2 Does entropy increase or decrease with increase in temperature Explain 3 A
The 1st ionisation energy H i of an element is the energy needed to remove one electron from each atom in one mole of atoms of the element in the gaseous state to form one mole of gaseous 1 ions Born Haber cycle is a particular type of enthalpy cycle used to calculate lattice energy Lattice energy of lithium fluoride H 1 obtained by Entropy Enthalpy and Gibbs Free Energy Practice Problems A summary practice problem set on chemical thermodynamics to cover examples of determining whether the process is spontaneous or not calculating the entropy of the reaction S the entropy change associated with phase transitions Gibbs free energy G of reaction spontaneity
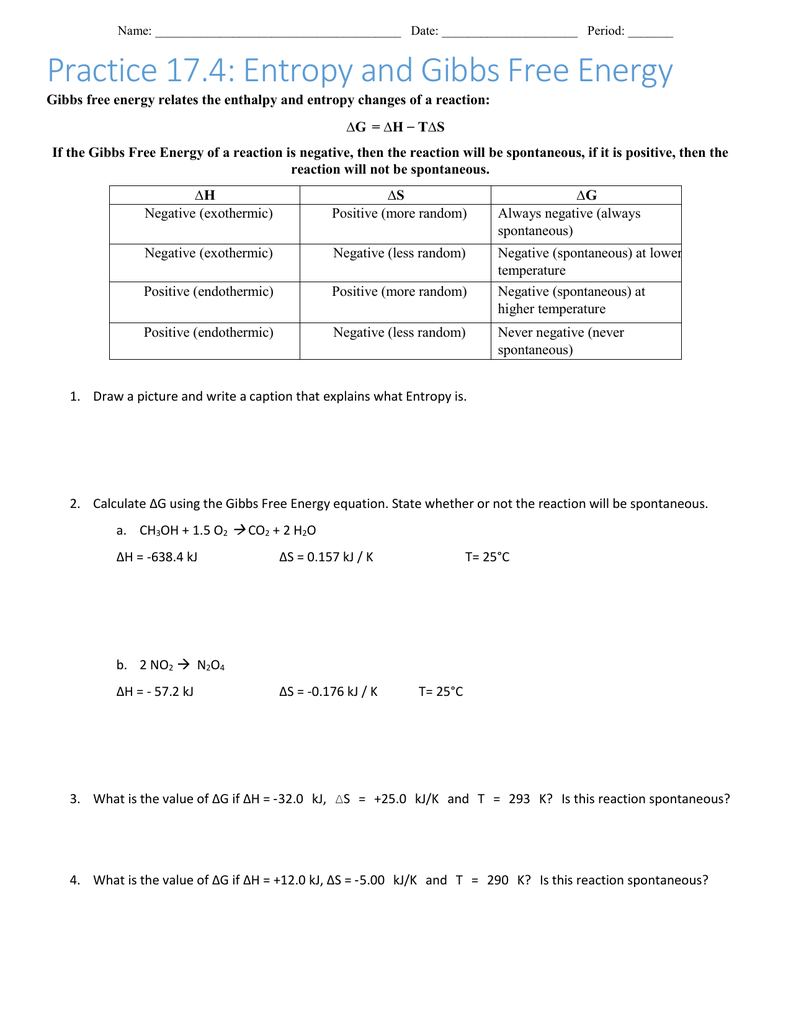
Gibbs Free Energy Worksheet Answer Key worksheet
https://s2.studylib.net/store/data/010175267_1-786f689c235fddbe44d1f426dc40cbc8.png

Gibbs Free Energy Worksheet Worksheets For Home Learning
https://static.docsity.com/documents_first_pages/2021/04/20/6f2ede12a877bf8a620baa97384bbc40.png
Entropy And Gibbs Free Energy Worksheet - WE 14 2 Entropy change of vaporization 3 on p 662 in Chemistry Calculate the entropy change when 1 00 mol of water at 0 C freezes to form ice For water fus Ho 6 02 kJ mol 1 Strategy Use Equation 14 6 which relates the entropy change for a system to the enthalpy change and the temperature Solution