Entropy And Free Energy Worksheet Worksheet on Entropy and Free Energy 1 Define entropy in your own words and list the variables or conditions that you must consider when comparing the entropy of two substances or when trying to determine the relative change in entropy Entropy is the degree of chaos or disorder in a system
Define Gibb s free energy enthalpy and entropy and the relationship between them Predict the sign of S in the following processes Briefly explain your answer 3 O2 g 2 O3 g H2O l H2O g 4 Al s 3 O2 g 2 Al2O3 s H2SO4 aq 2 H aq SO 2 4 aq S7 E 2a The freezing of water is a decrease in entropy so it has the smallest Delta S because solids have less entropy than liquids The other two options represent an increase in Entropy The sublimation of ice is a large increase in Entropy because gas has more Entropy than solids The sublimation of ice to gas is more of an increase than
Entropy And Free Energy Worksheet
Entropy And Free Energy Worksheet
https://media.cheggcdn.com/media/333/33367e8c-cf81-48a6-909d-e4892e3b8081/image
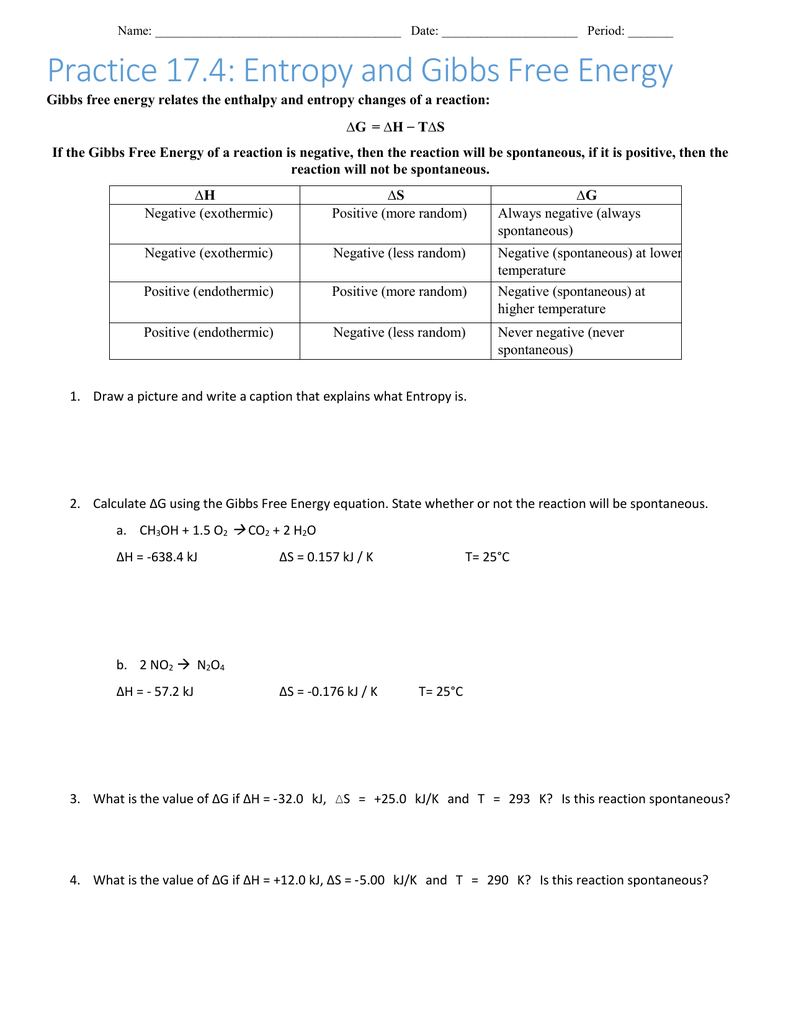
Worksheet 1
https://s2.studylib.net/store/data/010175267_1-786f689c235fddbe44d1f426dc40cbc8.png
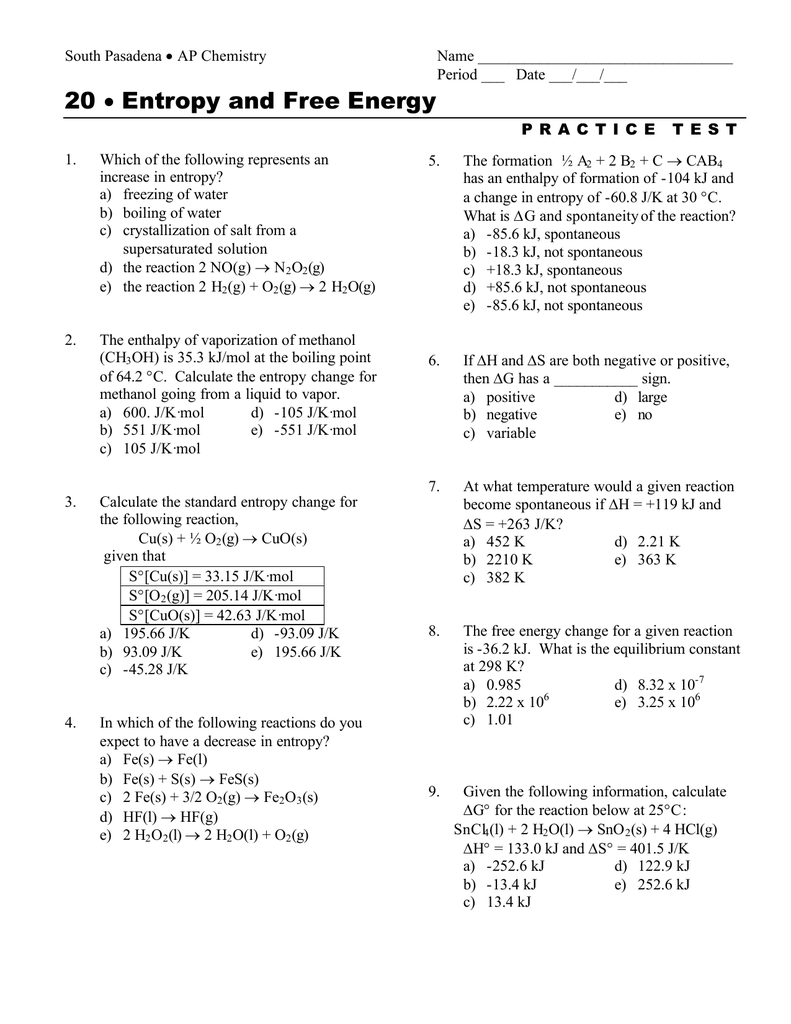
20 Entropy and Free Energy
https://s2.studylib.net/store/data/012945643_1-75c5b4d5b2c933c80bbd0ac0bbfbd015.png
A P Chemistry Practice Test Ch 16 Spontaneity Entropy and Free Energy MULTIPLE CHOICE Choose the one alternative that best completes the statement or answers the question 1 The thermodynamic quantity that expresses the degree of disorder in a system is A entropy B internal energy C heat flow D enthalpy E bond energy Calculate the change of the Gibbs free energy for the reaction at 25 C where standard free energy of formation of C 2 H 4 g H 2 O g C 2 H 5 OH l are 68 229 175 kJ mol respectively entropy change free energy change electrode cell potential equilibrium constant Answer a enthalpy change
Determine whether each reaction is spontaneous or not by calculating the Suniv based on the values of H rxn S rxn and T Assume that all the components in the reaction are in their standard states a H rxn 126 kJ S rxn 231 J K T 298 K b H rxn 5 kJ S rxn 139 J K T 298 K ENTROPY and GIBBS FREE ENERGY Entropy is the degree of randomness in a substance The symbol for change in entropy is S Solids are very ordered and have low entropy
More picture related to Entropy And Free Energy Worksheet
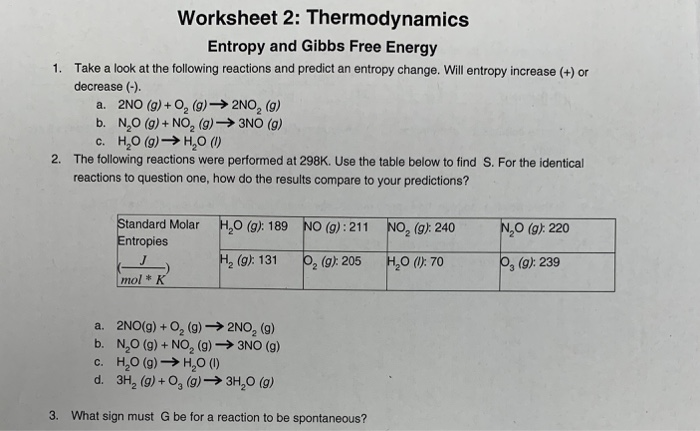
Solved Worksheet 2 Thermodynamics Entropy And Gibbs Free Chegg
https://media.cheggcdn.com/media/de8/de82b24e-44fc-40a3-ab37-d385e012ec7f/image.png

Gibbs Free Energy Worksheet
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/86c4f35503fa85cedcc7c38630538286/thumb_1200_1553.png

Entropy And Free Energy Worksheet For 11th Higher Ed Lesson Planet
https://content.lessonplanet.com/resources/thumbnails/193182/original/cgrmlwnvbnzlcnqymdezmdmzmc0yodc3ltvzodkyzs5qcgc.jpg?1414289556
Answer Exercise 8 E 3 Many plastic materials are organic polymers that contain carbon and hydrogen The oxidation of these plastics in air to form carbon dioxide and water is a spontaneous process however plastic materials tend to persist in the environment Explain Answer 8 2 Entropy Exercise 8 E 4 By looking at the change in enthalpy and the change in entropy as a result of a reaction we can quantitatively find out whether or not the reaction is spontaneous by introducing a new term Gibbs Free Energy Gibbs Free Energy Gibbs Free Energy is a new thermodynamic value that is used to determine the spontaneity of a reaction The formula
Key Worksheet 19 Spontaneity Entropy and Free Energy Free Energy Objectives To understand and apply the concept of free energy with respect to equilibria spontaneity and work Free Energy for an element in it s standard state is 0 For a process to be spontaneous DG for that process must be 0 Page 1 of 8 Entropy and Free Energy Worksheet Write the expression for the crystallization of sodium acetate trihydrate from its supersaturated solution 2 What is the sign of S for this change Explain 3 Write the expression the change in free energy G in terms of H and S
Chemistry Enthalpy And Gibbs free energy By GreenAPL Teaching
http://dryuc24b85zbr.cloudfront.net/tes/resources/11041090/image?width=500&height=500&version=1493910335878

Chemistry Blog Entropy Gibbs Free Energy Worksheet 4 10
https://lh4.googleusercontent.com/-nUrxx_yhQwk/U0a4Tq3v2fI/AAAAAAAAARE/bvKu0qneGgc/s640/blogger-image-1413012439.jpg
Entropy And Free Energy Worksheet - Determine whether each reaction is spontaneous or not by calculating the Suniv based on the values of H rxn S rxn and T Assume that all the components in the reaction are in their standard states a H rxn 126 kJ S rxn 231 J K T 298 K b H rxn 5 kJ S rxn 139 J K T 298 K

