empirical formula of mgo Magnesium oxide MgO or magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium see also oxide It has an empirical formula of MgO and consists of a lattice of Mg ions and O ions held together by ionic bonding Magnesium hydroxide forms in the presence of water MgO H2O Mg OH 2 but it can be reversed by heating it to remove moi
Learn how to determine the empirical formula of magnesium oxide by reacting magnesium metal with oxygen gas and measuring the masses of the reactants and products The empirical formula of magnesium oxide is MgO where Mg An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains The law of conservation of mass applies to closed and non enclosed
empirical formula of mgo
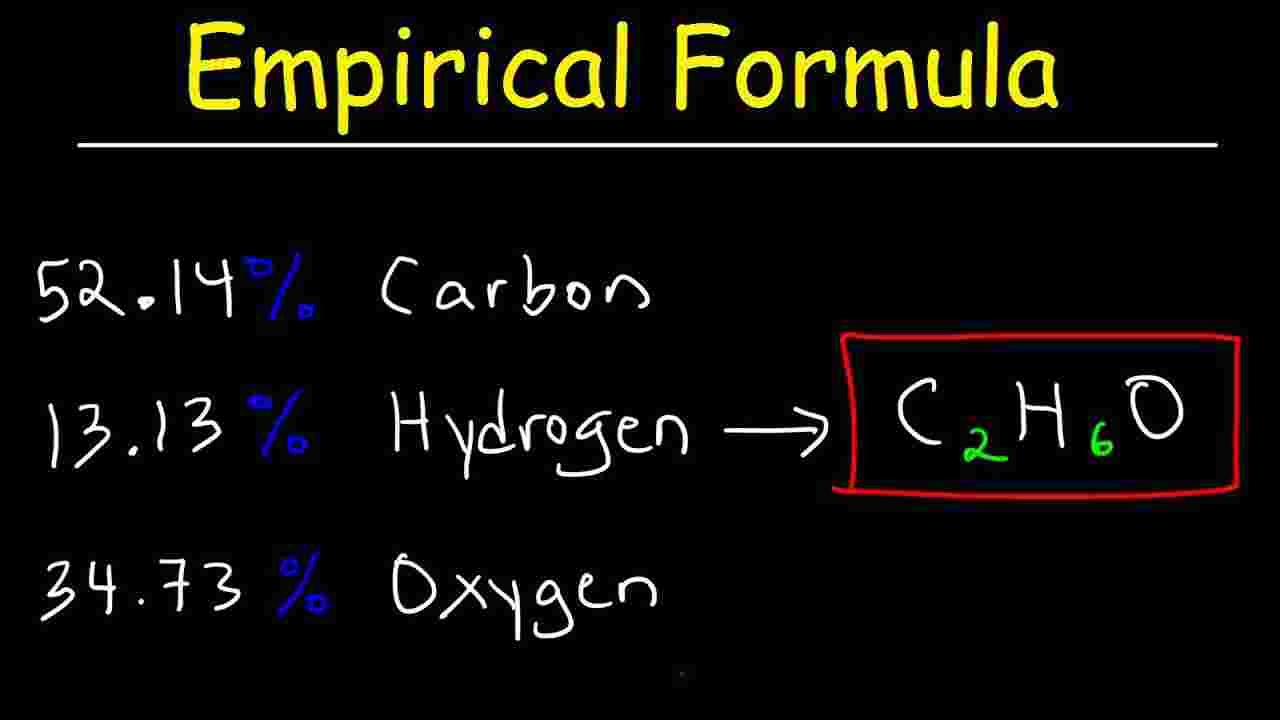
empirical formula of mgo
https://www.len.com.ng/upload_blog_attachment/15e342249f09383.97127923.jpg

Solved DATA AND OBSERVATIONS Determining The Empirical Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/0d0/0d08aa2e-3edf-491a-8d82-0fc53dd6ac37/phpf9Hzhy.png

111 Lab Report Experiment 5 Empirical Formula Goal To
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/14ec836990752a11dabb5feadda0b73c/thumb_1200_1553.png
Magnesium is reacted with oxygen from the air in a crucible and the masses before and after the oxidation are measured The resulting masses are used to calculate the experimental The empirical formula of a substance can be determined experimentally if we know the identities of the elements in the compound and the amount of each element in mass or moles In this
Take an accurately measured mass of magnesium metal burn it in crucible and carefully measure the mass of the crucible after the reaction the difference is the mass of The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO a table Before
More picture related to empirical formula of mgo

Empirical Formula Of MgO YouTube
https://i.ytimg.com/vi/FAY7yVuw6-c/maxresdefault.jpg

Empirical Formula Lab Conclusion Magnesium Oxide YouTube
https://i.ytimg.com/vi/Vpgb1Y321WA/maxresdefault.jpg
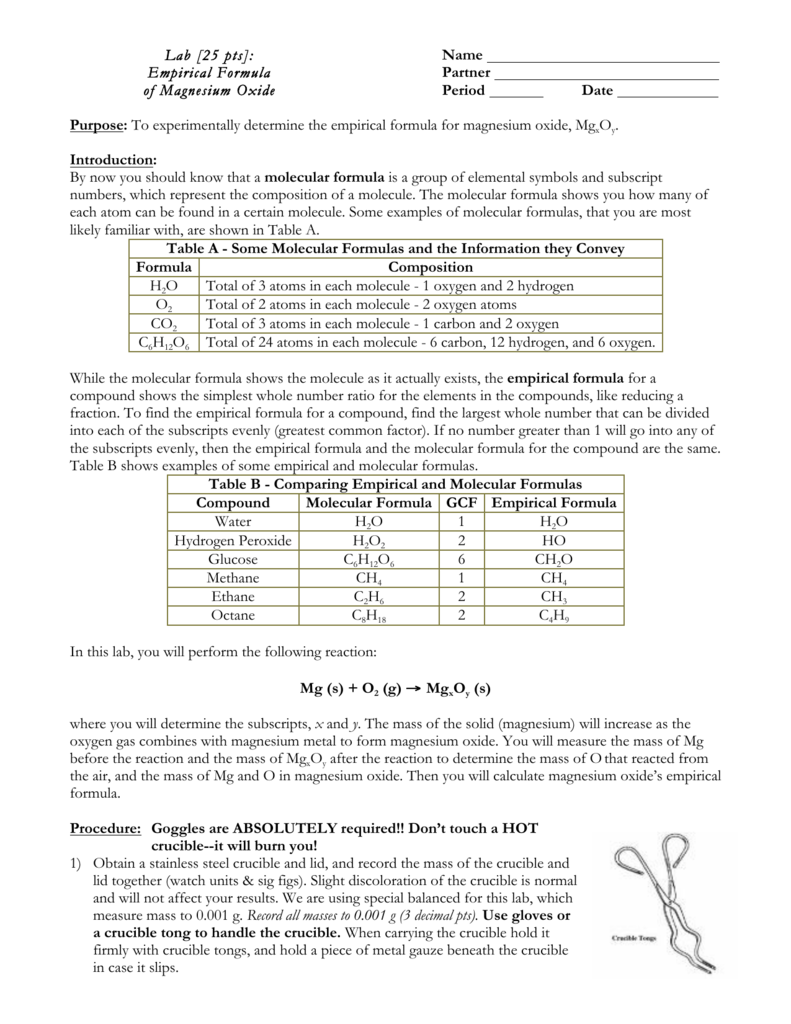
LAB Empirical Formula MgO
https://s3.studylib.net/store/data/008354760_1-049c4eb8b6fd42ef205190ac5239c7ab.png
DATA ANALYSIS 1 Determine the empirical formula of MgO based on your data Follow the steps used in the introduction for finding the empirical formula of NO 2 2 The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the
The empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound An example of this concept is the empirical formula of CH for From the masses of magnesium and oxygen that combine we can calculate the empirical formula of magnesium oxide We will weigh the magnesium before it combines with

Lab Report Empirical Formula Of MgO2 Lab Composition Of A Mixture
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/d2e36a07868353ef5f2c8b6d1618c3d9/thumb_1200_1698.png

EMPIRICAL FORMULA OF MgO YouTube
https://i.ytimg.com/vi/urMZGh3Vx9E/maxresdefault.jpg
empirical formula of mgo - Magnesium is reacted with oxygen from the air in a crucible and the masses before and after the oxidation are measured The resulting masses are used to calculate the experimental