empirical formula of magnesium oxide lab report The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO For today s
The experiment highlighted the formation of an ionic bond between a metal magnesium and a non metal oxygen to produce magnesium oxide The empirical formula indicated the simplest ratio of elements in the In this experiment the percent composition and empirical formula of magnesium oxide the main compound that is formed when magnesium metal combines with oxygen in air will be determined Heating magnesium in the presence of air
empirical formula of magnesium oxide lab report

empirical formula of magnesium oxide lab report
https://sp-uploads.s3.amazonaws.com/uploads/services/1144337/20210729115833_610297e96fd7e_lab_6_chm130ll_empirical_formula_of_magnesium_oxide_w_answerspage2.png

Empirical Formula Of Magnesium Oxide Lab Report Answers The
https://media.cheggcdn.com/study/cb3/cb358248-96d1-4a1a-b541-f492b094046c/image.png
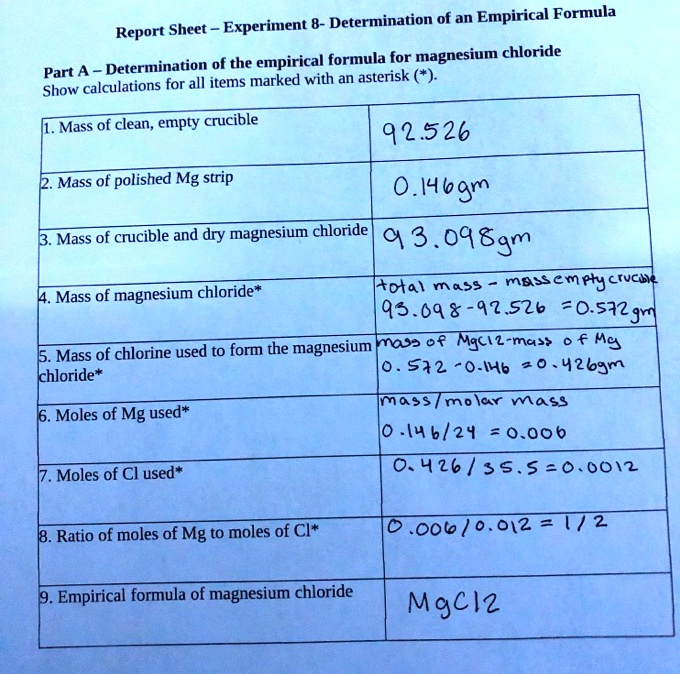
Magnesium Oxide Production Lab Lab Report 4 The Synthesis Of
https://cdn.numerade.com/ask_images/83334d07f28448d4a85f51346685803e.jpg
Michael Jansen reflects on a very common empirical formula lab that asks students to determine the empirical formula of MgxOy He then explains how he continues to use it as a successful failure how he Experiment 4 Determination of the Empirical Formula of Magnesium Oxide Introduction A molecular formula tells the number of atoms in the molecule such as H2O for water or H2O2
A combustion reaction carried out in a heated crucible using a Bunsen burner can be used to determine the empirical formula of magnesium oxide This lab demonstrates the law of The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide
More picture related to empirical formula of magnesium oxide lab report

DOC IB Chemistry IA Determining The Empirical Formula Of Magnesium
https://0.academia-photos.com/attachment_thumbnails/34358970/mini_magick20180815-12926-w0836a.png?1534400147
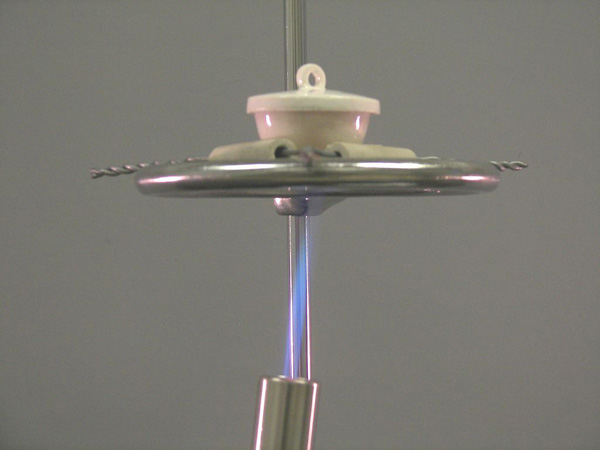
IS8025 Determination Of The Empirical Formula Of Magnesium Oxide
http://www.sciencelabsupplies.com/images/magictoolbox_cache_from_database/1b7f1df8ae54c4c23d8a23545c0993ae.jpg

Empirical Formula Of Magnesium Oxide Postlab Analysis YouTube
https://i.ytimg.com/vi/SjCVJxpIfg4/maxresdefault.jpg
The predicted empirical formula for magnesium oxide is 1 1 MgO because magnesium has a 2 charge and oxygen has a 2 charge In this experiment the empirical formula came out to 11 10 potentially meaning that not all of the Introduction The empirical formula for magnesium oxide was found in this experiment by oxidizing a weighed strip of magnesium The reaction of oxygen with magnesium increased the mass of the newly formed
The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO For today s AIM OF EXPERIMENT To determine the empirical formula of magnesium oxide PROBLEM STATEMENT What is the empirical formula of magnesium oxide MATERIALS

EXPERIMENT 7 EMPIRICAL FORMULA OF MAGNESIUM OXIDE
https://img.yumpu.com/26323063/1/500x640/experiment-7-empirical-formula-of-magnesium-oxide.jpg

Magnesium Oxide Percent Yield Lab Report SchoolWorkHelper
https://schoolworkhelper.net/wp-content/uploads/2020/03/mg-o-calculation.jpg
empirical formula of magnesium oxide lab report - Aim To calculate the empirical formula of magnesium oxide by heating magnesium ribbon in the presence of oxygen and measuring the mass change Materials