empirical formula of magnesium oxide lab report answers Study with Quizlet and memorize flashcards containing terms like Enter data from your lab and calculate the mass of your original magnesium sample Enter your answer to 1 decimal place
Experiment 7 Empirical Formulas Hypothesis When oxygen combines with magnesium during heating the mole ratio will be 1 1 creating the empirical formula to be MgO If KCLO 3 decomposes then KCl and O 2 will separate In this lab an experiment close to a combustion analysis was performed to find the empirical formula of Magnesium oxide A piece of magnesium was burned in a crucible The magnesium reacted with the nitrogen and oxygen in the air to
empirical formula of magnesium oxide lab report answers
empirical formula of magnesium oxide lab report answers
https://cdn.numerade.com/ask_images/83334d07f28448d4a85f51346685803e.jpg

LAB Empirical Formula MgO
https://s3.studylib.net/store/data/008354760_1-049c4eb8b6fd42ef205190ac5239c7ab-768x994.png

Stoichiometry Of Magnesium Oxide Lab
https://s3.studylib.net/store/data/008291385_1-90517373d86071c484407e43503116c3.png
In this experiment the percent composition and empirical formula of magnesium oxide the main compound that is formed when magnesium metal combines with oxygen in air will be determined Heating magnesium in the presence of air The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide
The empirical formula of magnesium oxide is MgO Here is a video that illustrates how to determine an empirical formula The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO a table Before
More picture related to empirical formula of magnesium oxide lab report answers

Magnesium Oxide Percent Yield Lab Report SchoolWorkHelper
https://schoolworkhelper.net/wp-content/uploads/2020/03/mg-o-calculation.jpg

Empirical Formula Lab Conclusion Magnesium Oxide YouTube
https://i.ytimg.com/vi/Vpgb1Y321WA/maxresdefault.jpg

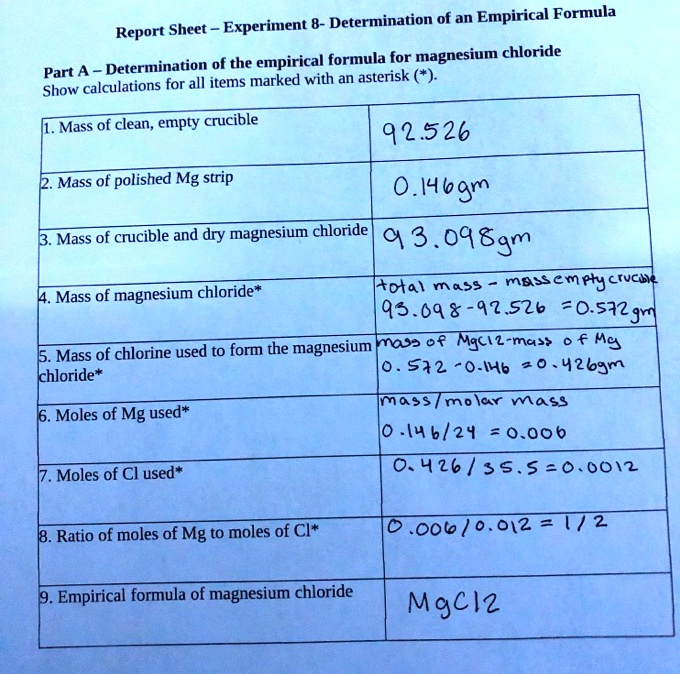
SOLVED Text Report Sheet Experiment 6 Empirical Formula Of Magnesium
https://cdn.numerade.com/ask_images/92512f47dd3d456db9200e0f1c271f13.jpg
The empirical formula of a substance can be determined experimentally if we know the identities of the elements in the compound and the amount of each element in mass or Magnesium oxygen magnesium oxide Rxn 1 From the masses of magnesium and oxygen that combine we can calculate the empirical formula of magnesium oxide We will weigh the
The empirical formula of a compound gives the lowest whole number ratio of the constituent atoms that is consistent with the mass ratios measured by experiment In this lab magnesium Michael Jansen reflects on a very common empirical formula lab that asks students to determine the empirical formula of MgxOy He then explains how he continues to

Empirical Formula Of Magnesium Oxide Lab Report Answers The
https://sp-uploads.s3.amazonaws.com/uploads/services/1144337/20210729115833_610297e96fd7e_lab_6_chm130ll_empirical_formula_of_magnesium_oxide_w_answerspage2.png

Magnesium Oxide Lab
https://chemistry.analia-sanchez.net/wp-content/uploads/Unorganized/mgo-lab-1024x853.png
empirical formula of magnesium oxide lab report answers - The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide