empirical formula of magnesium oxide lab errors Michael Jansen reflects on a very common empirical formula lab that asks students to determine the empirical formula of MgxOy He then explains how he continues to use it as a successful failure how he
Study with Quizlet and memorize flashcards containing terms like Enter data from your lab and calculate the mass of your original magnesium sample Enter your answer to 1 decimal place To find the experimental formula of Magnesium Oxide and compare it with the literature formula Procedure First we take a magnesium ribbon and rub or scrub it with rough paper in order to remove the existing magnesium oxide residue
empirical formula of magnesium oxide lab errors

empirical formula of magnesium oxide lab errors
https://d1e4pidl3fu268.cloudfront.net/ae63cff7-538b-4f22-b46f-09e8e0e15e6d/SC9a_Preview2.jpg

Magnesium Oxide Lab
https://chemistry.analia-sanchez.net/wp-content/uploads/Unorganized/mgo-lab-1024x853.png
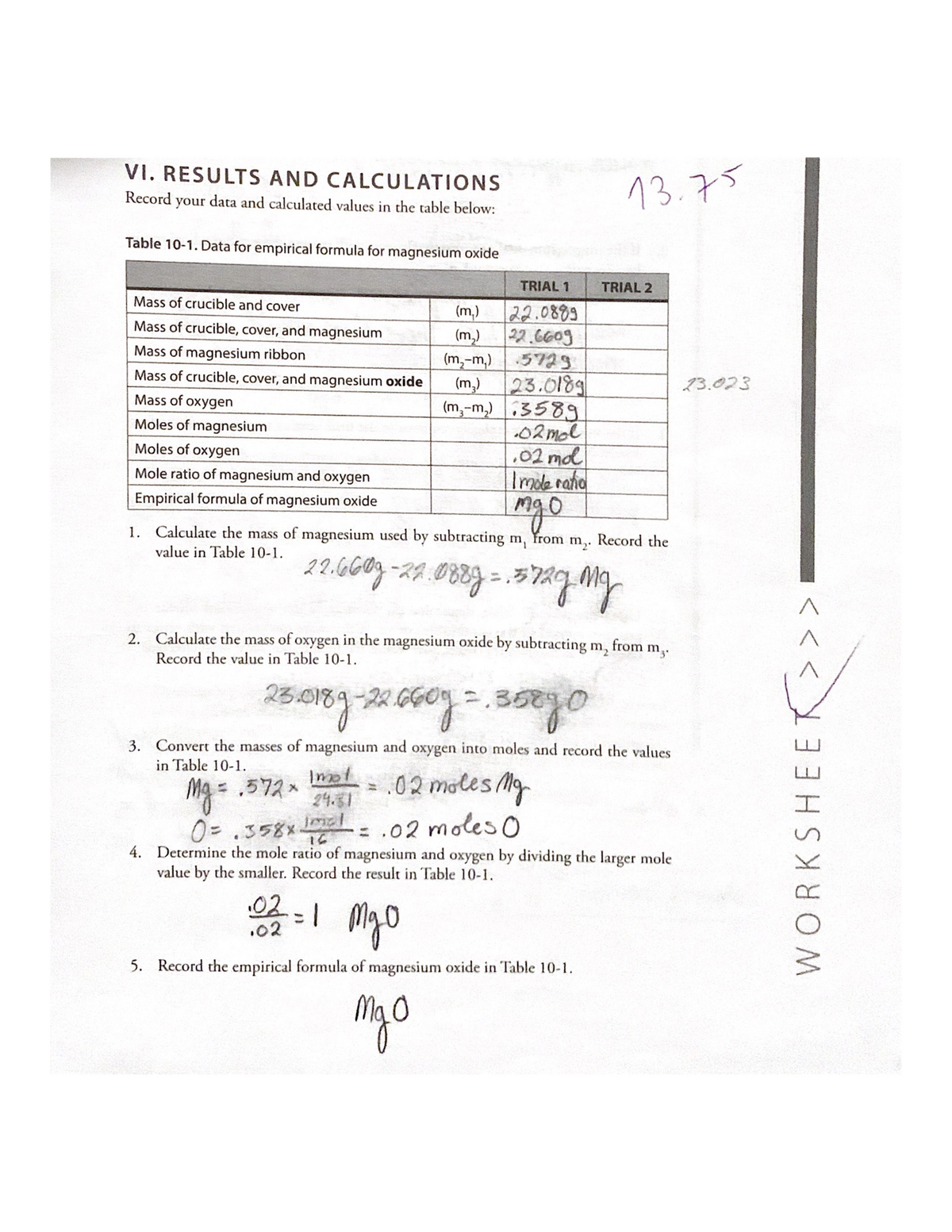
Lab 10 Determining The Empirical Formula Of Magnesium Oxide CHEM 100
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/dd8b97ee51bb1e39bb2b7828871bf433/thumb_1200_1553.png
They not only have to calculate the empirical formula of a compound quantitatively using mole conversions but they must also interpret their results suggest sources of error and reflect scientifically on the strengths and From the masses of magnesium and oxygen that combine we can calculate the empirical formula of magnesium oxide We will weigh the magnesium before it combines with the oxygen and we will also weigh the product of the reaction magnesium oxide
Calculations and conclusions on finishing the empirical formula lab The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence such as H2O for water or HO for hydrogen peroxide When magnesium and oxygen are heated together they react magnesium oxygen magnesium oxide Rxn 1 From the masses of magnesium and oxygen that combine we can calculate the
More picture related to empirical formula of magnesium oxide lab errors

How To Write Chemical Formula Of Magnesium Oxide YouTube
https://i.ytimg.com/vi/z2bwjaAN-8Q/maxresdefault.jpg

Empirical Formula Of Magnesium Oxide GCSE Lesson SC9a CC9a Teaching
https://d1e4pidl3fu268.cloudfront.net/0994085c-eac0-4371-84c2-62c4316ea053/SC9a_Cover.crop_691x518_12,0.preview.jpg

3 Experimental Determination Of Empirical Formula Of Magnesium Oxide
https://i.ytimg.com/vi/R9bUO70UfN8/maxresdefault.jpg
Three experimental errors that could affect the results include the potential escape of magnesium oxide fumes incomplete combustion of magnesium and inadequate firing of the crucible and lid To improve results Magnesium is reacted with oxygen from the air in a crucible and the masses before and after the oxidation are measured The resulting masses are used to calculate the experimental empirical formula of magnesium oxide which is then compared to the theoretical empirical formula
During this lab you will start with two separate elements and create a compound Using the mass of the elements that you begin with and the mass of the final product you should be able to determine the empirical formula of the compound magnesium oxide The goal of this experiment is to determine the empirical formula of magnesium oxide experimentally Objectives 1 To burn a sample of magnesium in air and measure the gain in mass 2 To determine the empirical formula of magnesium oxide 3 To find the theoretical and experimental yields of magnesium oxide and report statistics
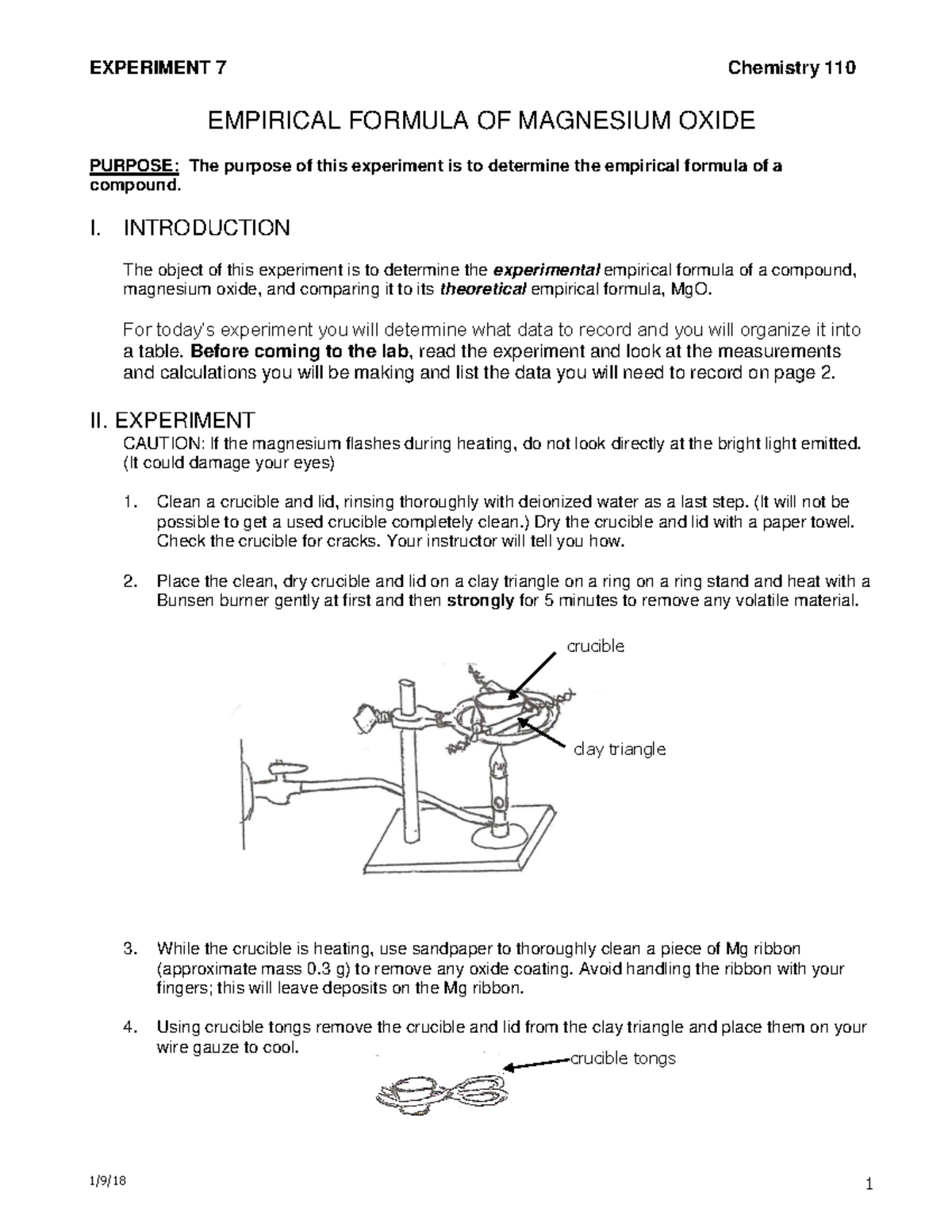
Chem 110 Exp 7 Empirical Formula Magnesium Oxide 2018 Crucible Clay
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/4f6367158f80b83577b0de9028c9b810/thumb_1200_1553.png

EXPERIMENT 7 EMPIRICAL FORMULA OF MAGNESIUM OXIDE
https://img.yumpu.com/26323063/1/500x640/experiment-7-empirical-formula-of-magnesium-oxide.jpg
empirical formula of magnesium oxide lab errors - From the masses of magnesium and oxygen that combine we can calculate the empirical formula of magnesium oxide We will weigh the magnesium before it combines with the oxygen and we will also weigh the product of the reaction magnesium oxide