empirical formula of magnesium oxide lab data table The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO For today s
The empirical formula of a compound gives the lowest whole number ratio of the constituent atoms that is consistent with the mass ratios measured by experiment In this lab magnesium A combustion reaction carried out in a heated crucible using a Bunsen burner can be used to determine the empirical formula of magnesium oxide This lab demonstrates the law of
empirical formula of magnesium oxide lab data table

empirical formula of magnesium oxide lab data table
https://media.cheggcdn.com/study/a7c/a7c4c45c-73e9-4f0b-b87b-a6e5c37402c9/image.png
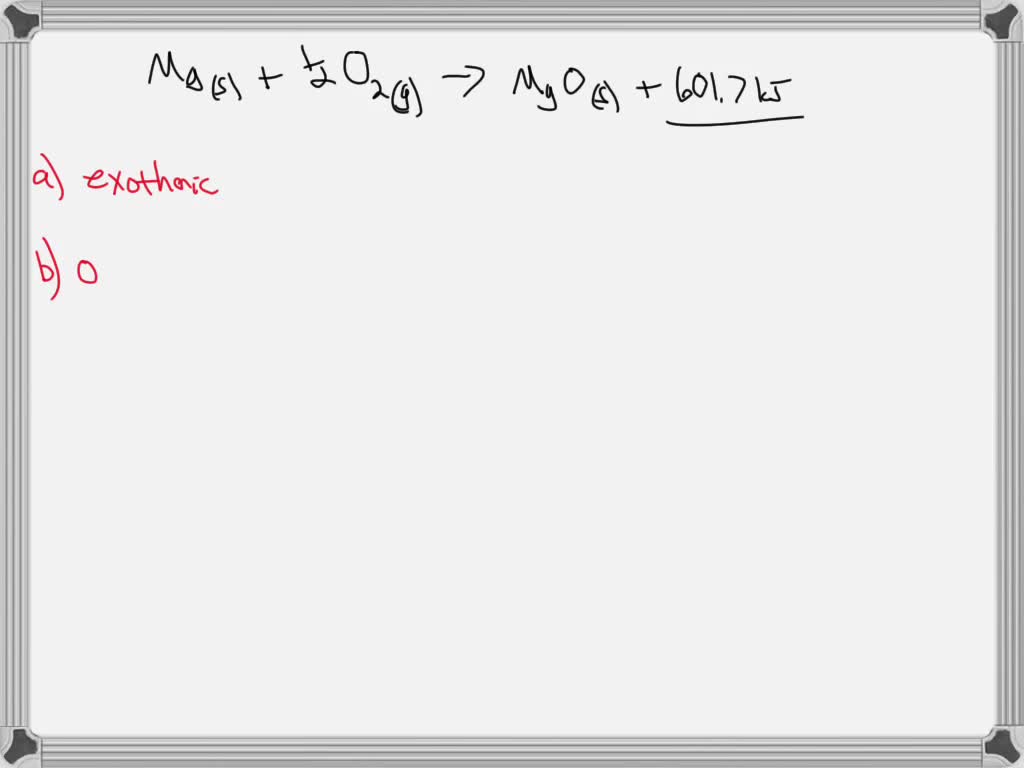
The Formation Of Magnesium Oxide MgO Is Shown Below SolvedLib
https://cdn.numerade.com/ask_previews/fac5f521-4535-4701-8a05-78a79ea9c21b_large.jpg

Empirical Formula Of Magnesium Oxide Lab Report Answers The
https://sp-uploads.s3.amazonaws.com/uploads/services/1144337/20210729115833_610297e96fd7e_lab_6_chm130ll_empirical_formula_of_magnesium_oxide_w_answerspage2.png
Experiment 4 Determination of the Empirical Formula of Magnesium Oxide Introduction A molecular formula tells the number of atoms in the molecule such as H2O for water or The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide
The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide In this experiment the empirical formula of magnesium oxide will be found by reacting magnesium with oxygen to produce magnesium oxide The mass of the reactant
More picture related to empirical formula of magnesium oxide lab data table

The Empirical Formula Of Magnesium Oxide Lab A Successful Failure
https://www.chemedx.org/sites/www.chemedx.org/files/images/blog/deanna-cullen/empirical-formula-magnesium-oxide-lab-successful-failure-next-steps—and-very-important-lesson.png

Determination Of The Empirical Formula Of Magnesium Oxide
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/3c2cdc4e918603381ff40181bdd62999/thumb_1200_1553.png

Magnesium Oxide Lab
https://chemistry.analia-sanchez.net/wp-content/uploads/Unorganized/mgo-lab-1024x853.png
Michael Jansen reflects on a very common empirical formula lab that asks students to determine the empirical formula of MgxOy He then explains how he continues to use it as a successful failure how he Empirical formula of magnesium oxide is written with the symbol for magnesium Mg written before the symbol for oxygen O using the lowest whole number ratio of moles of magnesium x to moles of oxygen y the
The object of this experiment is to determine the experimental empirical formula of a compound magnesium oxide and comparing it to its theoretical empirical formula MgO For today s Based on your experimental data write the empirical formula for magnesium oxide After you find the answer to this question place the results of your empirical formula on the board as

Magnesium Oxide Lab
https://s2.studylib.net/store/data/026038291_1-3ae37768452a774ed0307a5bd579f517-768x994.png
Empirical Formula Of Magnesium Oxide Lab Report Answers The
https://studymoose.com/wp-content/uploads/essay-thumbnails/determining-the-empirical-formula-of-magnesium-oxide-lab-post-preview.webp
empirical formula of magnesium oxide lab data table - The empirical formula of magnesium oxide Mg x O y is written as the lowest whole number ratio between the moles of Mg used and moles of O consumed This is found by determining the moles of Mg and O in the product divide