empirical formula of magnesium and sulfur We divide thru by the smallest molar quantity 0 958 mol to get and empirical formula of M gSO3 magnesium sulfite Ordinarily you would not be given the percentage of
When an ionic compound is formed from magnesium and oxygen the magnesium ion has a 2 charge and the oxygen atom has a 2 charge Although both of these ions have higher charges than the ions in lithium bromide they still Determine the empirical formula for a compound containing magnesium and sulfur
empirical formula of magnesium and sulfur

empirical formula of magnesium and sulfur
https://www.jmamg.com/uploadfiles/2022/08/20220811193702016.jpg?MS0xMC03LmpwZw==

Empirical Formula Of Magnesium Oxide Chemistry Classes Ronald
https://chemistry.analia-sanchez.net/wp-content/uploads/Unorganized/mgo-lab-1024x853.png

Question Video Calculating The Relative Formula Mass Of Magnesium
https://media.nagwa.com/480139267385/en/thumbnail_l.jpeg
An ionic formula like ce NaCl is an empirical formula This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions You then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula We will start with binary ionic which are between monatomic anions and cations
Magnesium Sulfide has a formula of MgS Magnesium is a metal cation with a charge of Mg 2 Sulfur is a nonmetal anion with a charge of S 2 In order to bond Word Equation Magnesium Sulfur Magnesium Sulfide Mg S MgS is a Synthesis reaction where one mole of Magnesium Mg and one mole of Sulfur S combine to form one
More picture related to empirical formula of magnesium and sulfur

Empirical Formula Of Magnesium Oxide
https://cdn.numerade.com/ask_images/c6afc5e67eb340ab87dc930090221659.jpg

Journal Of Magnesium And Alloys
https://www.jmamg.com/uploadfiles/2023/02/20230222102037066.jpg?MS0xMS0xLmpwZw==
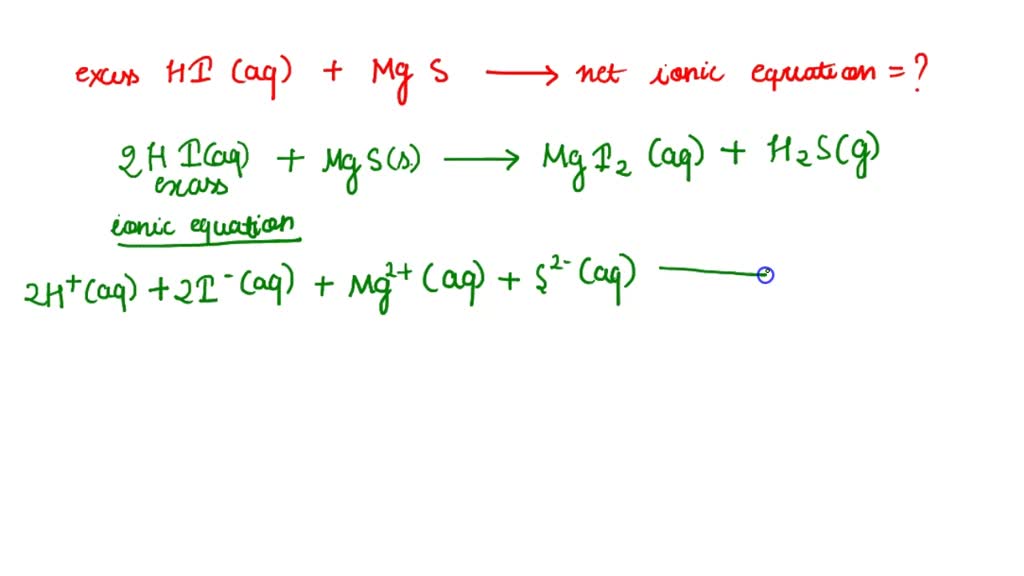
SOLVED Write Net Ionic Equation For The Reaction That Occurs When
https://cdn.numerade.com/ask_previews/33ecb549-dd87-4d9c-82c2-0e2ee045ef1e_large.jpg
Convert the mass of each element to moles using the atomic masses from the periodic table Divide the moles of each element by the smallest number of moles calculated Round to the nearest whole number to find the atom ratio which Calculation techniques for the higher tier to determine the empirical and molecular formulae of a substance masses of a required substance in a reaction and calculations involving moles
Calculate the empirical formula of the compound with a percent composition of 23 3 Magnesium Mg 46 Oxygen O 30 7 Sulphur Sulfur S Magnesium and nitrogen react to form an ionic compound Predict which forms an anion which forms a cation and the charges of each ion Write the symbol for each ion and name them

Empirical Formula Of Magnesium Oxide Postlab Analysis YouTube
https://i.ytimg.com/vi/SjCVJxpIfg4/maxresdefault.jpg

Which Form Of Magnesium Should You Take WhyNotNatural
https://whynotnatural.com/cdn/shop/articles/Comparison_of_magnesium_forms_and_purposes_including_l-threonate_glycinate_taurate_and_malate_1200x1200.png?v=1674244032
empirical formula of magnesium and sulfur - Word Equation Magnesium Sulfur Magnesium Sulfide Mg S MgS is a Synthesis reaction where one mole of Magnesium Mg and one mole of Sulfur S combine to form one