difference between molar and molal elevation constant Molar elevation constant Molar elevation constant can be defined as the elevation in boiling point produced when one mole of solute is dissolved in 1 kg i e 1000 g of
We can express the relationship between T b and concentration as follows T b mK b label eq2 where m is the concentration of the solute expressed in molality and K b is the molal boiling point elevation Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution The molal boiling point elevation constant is equal to the
difference between molar and molal elevation constant

difference between molar and molal elevation constant
https://qsstudy.com/wp-content/uploads/2015/11/Molar-Solution-and-Molal-Solution-1.jpg

Calculate The Molal Elevation Constant Of Water If Molar Enthalpy Of
https://hi-static.z-dn.net/files/d6e/9db6b68277203e0f2cc769720567beb4.jpg

Difference Between Molarity And Molality YouTube
https://i.ytimg.com/vi/BKysrjE0mKc/maxresdefault.jpg
According to Table PageIndex 1 the molal boiling point elevation constant for water is 0 51 C m Thus a 1 00 m aqueous solution of a nonvolatile molecular solute such as Boiling point of solution boiling point of pure solvent boiling point elevation T b The elevation in boiling point T b is proportional to the concentration of the solute in the solution It can be calculated via the following equation T b
Where K b is the boiling point elevation constant or the ebullioscopic constant and m is the molal concentration molality of all solute species Boiling point elevation constants are Molar Mass from Boiling Point Elevation or Freezing Point Depression The addition of a nonvolatile solute to a solvent causes the boiling point of the solvent to increase and the freezing point of the solvent to decrease molar mass
More picture related to difference between molar and molal elevation constant

Difference Between A 1 0 Molar Solution And A 1 Molal Solution
https://www.differencebetween.com/wp-content/uploads/2011/05/Difference-Between-a-1.0-Molar-Solution-and-a-1-Molal-Solution-fig-1-1024x678.jpg

Calculate The Value Of Molal Elevation Constant Water Delta S
https://haygot.s3.amazonaws.com/questions/1743844_1742634_ans_43515b24802848289c2aaa5fb3b88e52.jpg

SOLVED The Molal Elevation Constant Of Water Is 0 51 The Boiling Point
https://cdn.numerade.com/previews/4a5f20a8-35c7-4c1a-b23a-62074619223a_large.jpg
Molal elevation constant may be defined as the boiling point elevation produced when 1 mole of solute is dissolved in one kg 1000 g of the solvent If the mass of the solvent W is given in Molal elevation constant K b is defined as the elevation in boiling point of a solution when one mole of a non volatile solute is dissolved in one kilogram of a volatile solvent
The molal elevation constant for water is 0 513 o C kg mol When 0 2 mole of sugar is dissolved in 250 g of water calculate the temperature at which the solution boils under atmospheric pressure What is the molal elevation co Hint The molal elevation constant is related to the elevation in the boiling point of a solvent It can be derived in consideration with the molal concentration of
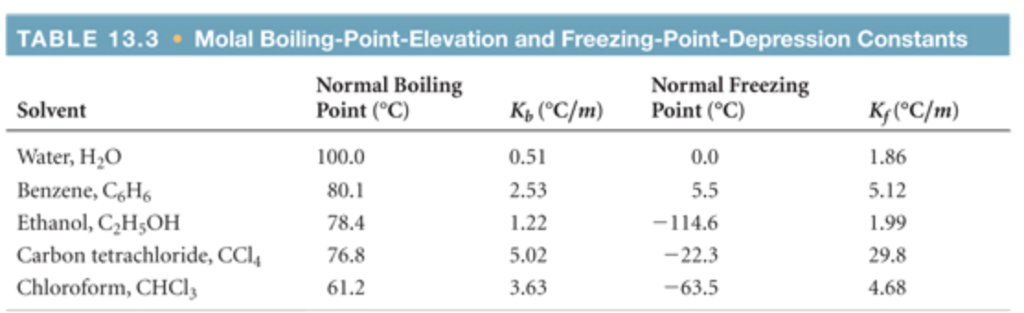
Solved TABLE 13 3 Molal Boiling Point Elevation And Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/c25/c2598e17-d027-445c-9256-407acd4e82cb/phpz1EA23.png

The Molal Elevation Constant For Water Is 0 56 K Kg Mol 1 The Boiling
https://hi-static.z-dn.net/files/d52/77f5d8a0c4df62a731eec976d5698cd2.jpg
difference between molar and molal elevation constant - Where K b is the boiling point elevation constant or the ebullioscopic constant and m is the molal concentration molality of all solute species Boiling point elevation constants are