article 10 2 of directive 2001 83 ec 3 The quality safety and efficacy requirements of proprietary industrially prepared medicinal products derived from human blood or plasma were ensured through
Dossier requirements Detailed pharmaceutical non clinical and clinical data required CTD format Specified in Annex I of Directive 2001 83 EC Further clarified in scientific Consolidated text Directive 2001 83 EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products
article 10 2 of directive 2001 83 ec

article 10 2 of directive 2001 83 ec
https://www.researchgate.net/publication/345182027/figure/fig1/AS:972360505118720@1608840218449/The-waste-policy-defined-in-the-Directive-2008-98-EC-EP-and-of-the-Counci-2018.png

Directive 2001 83 EC DIRECTIVE 2001 83 EC OF THE EUROPEAN PARLIAMENT
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/7ff25eba23a8c8ce10a6740144843099/thumb_1200_1698.png

2001 20 EC A European Directive E Book Frohberg
https://www.frohberg.de/product/Ciando423206/b/2001-20-ec-a-european-directive.jpg
DIRECTIVE 2001 83 EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 6 November 2001 on the Community code relating to medicinal products Directive 2001 83 EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use
Article 10a By way of derogation from Article 8 3 i and without prejudice Directive 2001 83 EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use
More picture related to article 10 2 of directive 2001 83 ec

Sample Directive Letters Format Examples And How To Write Directive
https://www.aplustopper.com/wp-content/uploads/2021/05/Ministerial-Directive-Letter.png
Directive 2001 95 Ec Of The European Parliament And Of The Council Of 3
https://imgv2-1-f.scribdassets.com/img/document/360657533/original/c5074a5567/1592895624?v=1
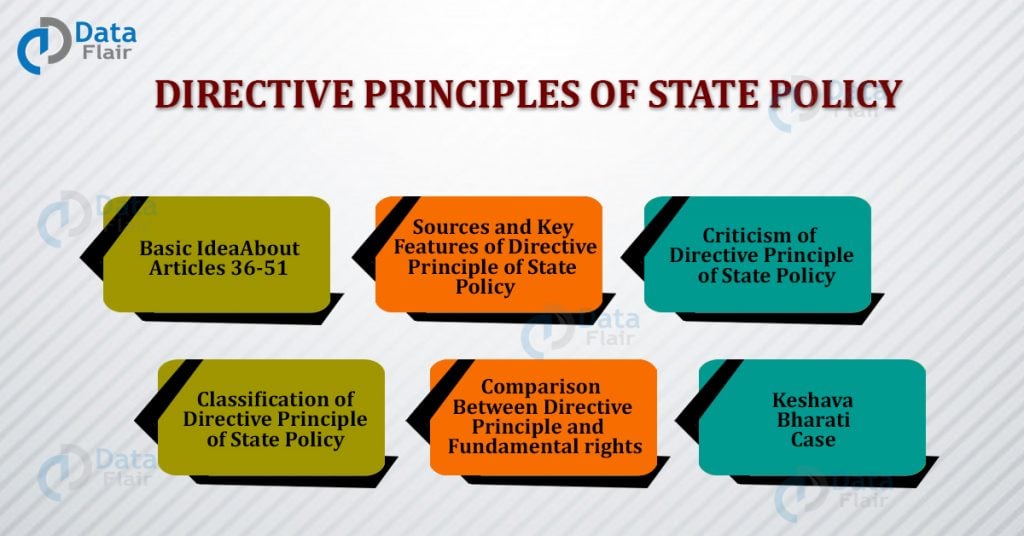
Directive Principles Of State Policy Articles 36 51 In Indian
https://data-flair.training/blogs/wp-content/uploads/sites/2/2020/08/Directive-principle-of-state-policy-1024x536.jpg
Directive 2001 83 EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use European Union In this context it is necessary to set EU wide standards for the manufacturing of active substances and to harmonise the implementation and enforcement of these standards
Directive 2001 83 EC of the European Parliament and of the Council of 6 November 2001 on the Community code relates to medicinal products for human use in mainly countries A marketing authorisation for the reference medicine has been granted on the basis of a complete dossier in accordance with article 8 3 10a 10b or 10c of

Directive Principles Of State Policy DPSP Meaning Articles
https://s3-ap-south-1.amazonaws.com/adda247jobs-wp-assets-adda247/articles/wp-content/uploads/2022/12/19203503/Directive-Principles-of-State-Policy.jpg

Assessment Report On Cucurbita Pepo L Semen Based On Article 16D 1
https://data.docslib.org/img/1591667/assessment-report-on-cucurbita-pepo-l-semen-based-on-article-16d-1-article-16f-and-article-16h-of-directive-2001-83-ec-as-amended-traditional-use.jpg
article 10 2 of directive 2001 83 ec - Article 10a By way of derogation from Article 8 3 i and without prejudice
