500 ml of 10 5 m naoh is mixed Answer 9 47 Solution 9 47 500 ml of 10 5 M NaOH is mixed with 500 ml of 2 5 10 5 M Ba OH 2 Calculate pH Given log 3 0 47 Check Answer and Solution for above questio
500 ml of 0 2 M H 2 S O 4 are mixed with 250 ml of 0 1 M B a O H 2 the normality of resulting solution is S 2 224 10 2 0 0223 moles litre 1 No of moles of Ca2 ions in 500 ml of solutions 0 011 Now when 500 ml of saturated solution is mixed with 500 ml of 0 4 M NaOH the
500 ml of 10 5 m naoh is mixed

500 ml of 10 5 m naoh is mixed
https://img.homeworklib.com/questions/e0414120-deaa-11eb-b81d-c9f11bbee24d.png?x-oss-process=image/resize,w_560
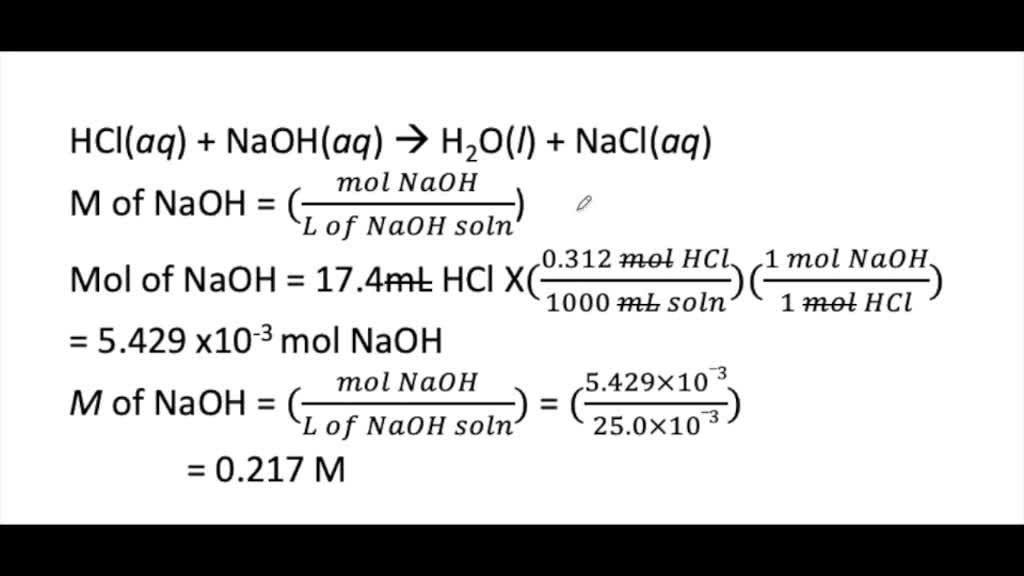
SOLVED Calculate The Concentration in Molarity Of An mathrm NaOH
https://cdn.numerade.com/previews/da120087-786d-4a12-b598-2457ac432c27_large.jpg

Click To See Additional Instructions A Student Standardized An NaOH
https://img.homeworklib.com/questions/57c7fd70-d3d1-11eb-b349-c5fa358dd02b.png?x-oss-process=image/resize,w_560
500 ml of 10 5 M NaOH is mixed with 500 ml of 2 5 x 10 5 M Last updated 6 29 2023 500 ml of 10 5 M NaOH is mixed with 500 ml of 2 5 x 10 5 M of Ba OH To the Calculator for Making Sodium Hydroxide Solution This calculator calculates the amounts of two starting solutions of given concentration needed to be mixed together to obtain the final
We mix 45 0 mL of 0 0675 M H 3 A solution with 28 0 mL of 0 0985 M NaOH What is the pH of the resulting solution Solution using millimoles 1 Write the chemical reaction H 3 A More than just an online dilution calculator Wolfram Alpha is a great tool for calculating the molarity molality mass fraction and amount fraction concentration of solutions It also generates solution properties and preparation recipes
More picture related to 500 ml of 10 5 m naoh is mixed

Calculate The Normality Of NaOH Solution Formed By Dissolving 0 2g NaOH
https://ph-static.z-dn.net/files/d43/72b42df963dd63c9503f6ac798856c9e.png

Solved If 10 0 ML Of A 10 0 M Stock Solution Of NaOH Is Diluted Of
https://files.transtutors.com/book/qimg/f9f1cb85-1e13-4659-aae0-5e2279342f76.png

OneClass 11 5 ML Of 0 470 M NaOH Is Mixed With 33 5 ML Of 0 34 M LiOH
https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/130/13011394.png
How to Calculate Units of Concentration Once you have identified the solute and solvent in a solution you are ready to determine its concentration Concentration may be expressed several different ways using percent Multiply the molarity of the strong base NaOH by the volume of the NaOH MB VB 0 500 M 20 70 mL Divide this answer 10 35 M mL by the volume of the acid HCl 0 15 mL MA MB VB VA 0 500 M 20 70
Dilute Solution of Known Molarity The solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration Solution 1 Determine how many moles of NaOH are present 4 00 mmol mL 5 00 mL 20 0 mmol Since NaOH is a strong base we have 20 0 mmol of OH ion 2 Since the pH of the
Solved H2SO4 NaOH Na2SO4 H20 Left H 3 H 2 S 1
https://www.coursehero.com/qa/attachment/21548860/
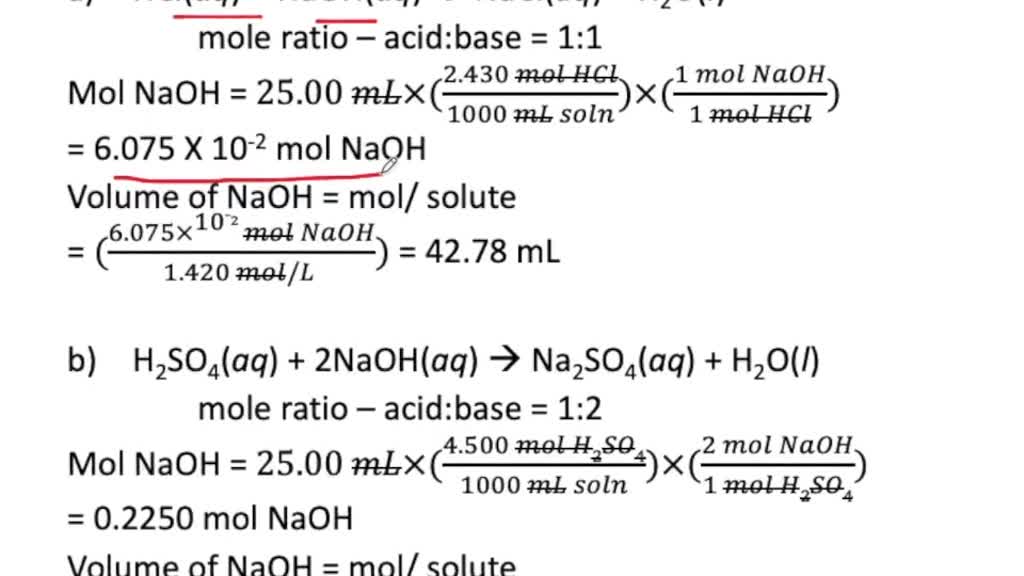
SOLVED Calculate The Volume Necessary in Milliliters To Provide The
https://cdn.numerade.com/previews/61b024de-0ae3-4451-8015-3b52cd24e69f_large.jpg
500 ml of 10 5 m naoh is mixed - What volume of a 5 0 M NaCl stock solution is necessary to prepare 500 mL of normal saline solution 0 16 M NaCl Answer 16 mL